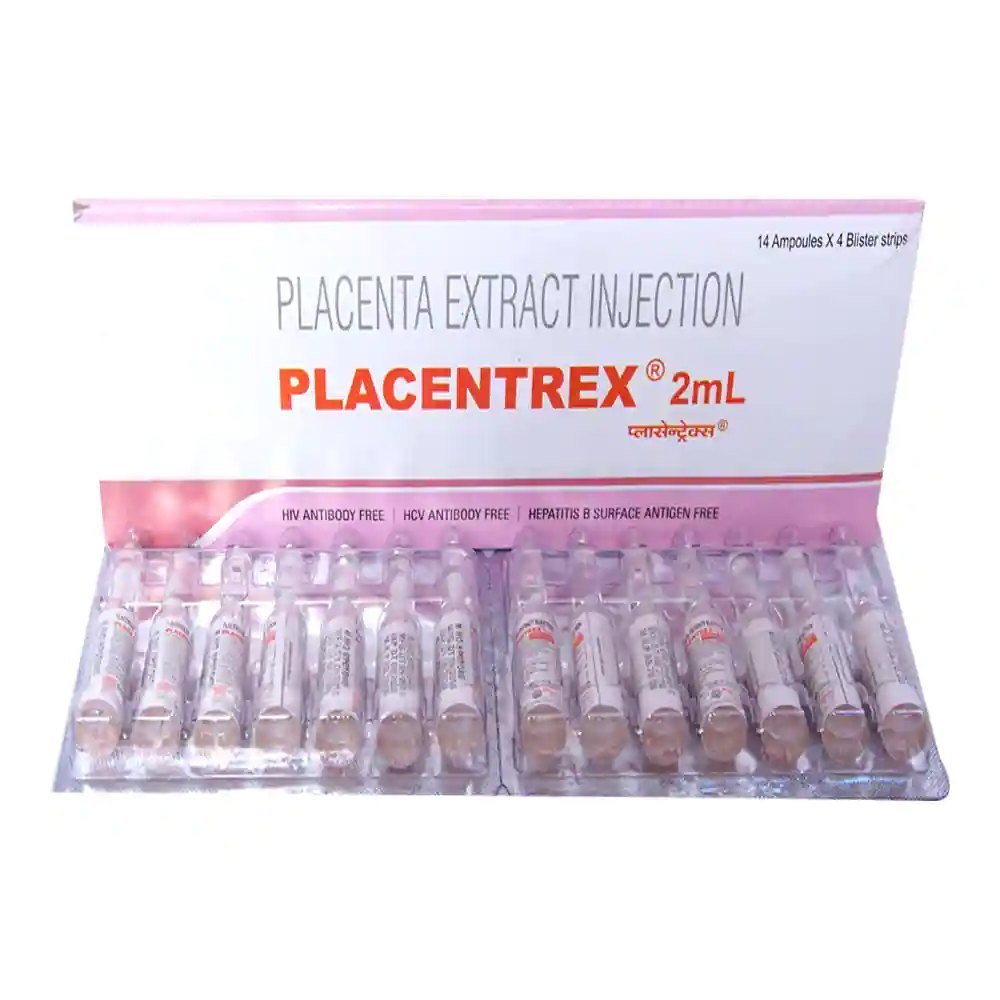
Placentrex Injection
✅ Wound healing promotion
✅ Tissue regeneration
✅ Skin rejuvenation
✅ Accelerated healing process
✅ Enhanced collagen production
Placentrex Injection contains Placenta extract.
Product Overview
Placentrex Injection is a therapeutic formulation containing biologically active Placenta extract as its key component. Presented in sterile ampoules, this medication is utilized for multiple clinical applications. The placental extract is obtained from mammalian sources and contains a rich profile of bioactive substances including growth factors, cytokines, and essential amino acids. By leveraging the regenerative potential of placental tissue, Placentrex Injection facilitates tissue restoration, accelerates wound healing, and promotes cellular regeneration across various medical indications.
Clinical Applications
In dermatological practice, Placentrex Injection demonstrates efficacy in managing chronic wounds, ulcerative conditions, burn injuries, and various cutaneous lesions. Gynecological applications include therapeutic management of cervical erosion and certain fertility-related conditions. The injection also finds utility in orthopedic settings for addressing musculoskeletal trauma and inflammatory joint pathologies. These diverse clinical benefits are attributed to the formulation’s capacity to enhance tissue regeneration, promote neovascularization, and stimulate collagen production.
Administration Guidelines
Placentrex Injection is delivered through intramuscular or intralesional routes as determined by the treating physician. Strict aseptic technique must be observed during administration, with thorough cleansing and disinfection of the injection site. Therapeutic protocols including dosage frequency and treatment duration are customized according to disease severity and individual patient response. Strict adherence to medical supervision is imperative for optimal therapeutic outcomes.
Mechanism of Action
The therapeutic effects of Placentrex Injection stem from its complex composition of bioactive placental constituents with regenerative and immunoregulatory properties. While the complete pharmacological profile continues to be elucidated, current understanding suggests multimodal mechanisms including stimulation of cellular proliferation (particularly fibroblasts and endothelial cells), enhancement of vascular network formation, and promotion of extracellular matrix synthesis. These combined actions facilitate comprehensive tissue repair and regeneration processes.
Dosage Protocol
Therapeutic regimens for Placentrex Injection are individualized based on clinical indication, disease progression, and therapeutic response. Administration is exclusively performed by qualified medical professionals through either intramuscular or intralesional routes. Precise dosing parameters including injection frequency and treatment duration are established by the attending physician following comprehensive clinical evaluation. Compliance with prescribed protocols is essential for achieving desired therapeutic endpoints.
Therapeutic Advantages
Placentrex Injection offers significant clinical benefits through its capacity to accelerate tissue repair mechanisms, thereby reducing healing time for various pathological conditions. The formulation demonstrates particular efficacy in complex wound management, potentially reducing complication risks in chronic ulcerative and burn cases. Additional benefits include improved surgical recovery outcomes and enhanced quality of life for patients across multiple medical specialties.
Adverse Effects
Transient local reactions including injection site discomfort, erythema, or mild edema may occur following administration. These typically resolve spontaneously without intervention. Rare hypersensitivity manifestations may include cutaneous reactions or respiratory distress, necessitating immediate medical attention. Patients should report any persistent or concerning symptoms to their healthcare provider.
Precautions
Comprehensive medical history disclosure including allergy profiles, concurrent medications, and pre-existing conditions is mandatory prior to initiation of therapy. Contraindications include known hypersensitivity to placental derivatives or any excipients in the formulation. Special consideration is required for pregnant or lactating patients, with therapy initiation only following thorough risk-benefit assessment by the treating physician.
Storage Conditions
Maintain Placentrex Injection at controlled room temperature (15-30°C), protected from light exposure and moisture. The product must not be subjected to freezing temperatures. Visual inspection prior to use is mandatory – discard any ampoules with compromised integrity or showing solution discoloration. Proper disposal of unused medication should follow biomedical waste management protocols.
Disclaimer:
The information provided herein represents carefully curated, medically reviewed content intended for educational purposes only. This material should not be construed as a substitute for professional medical advice, diagnosis, or treatment. The complete safety profile, potential drug interactions, and contraindications may not be fully detailed. We strongly recommend consultation with qualified healthcare providers for personalized medical guidance. This content aims to facilitate informed patient-physician dialogue rather than replace clinical judgment.
| Strength | 2 ml |
|---|---|
| Quantity | 7 Ampoule/s, 14 Ampoule/s, 21 Ampoule/s, 28 Ampoule/s |
 Placentrex Injection
Placentrex Injection













Reviews
There are no reviews yet