Tadapox is a combination medication that contains two active ingredients: Tadalafil and Dapoxetine. Tadalafil is used to treat erectile dysfunction (ED) by increasing blood flow to the penis, while Dapoxetine is used to treat premature ejaculation (PE) by delaying ejaculation. Together, they provide a dual-action treatment for men experiencing both conditions. Tadapox is typically taken as needed, approximately 30-60 minutes before sexual activity. It is important to follow the prescribed dosage and consult a healthcare provider before use, especially if you have underlying health conditions or are taking other medications.
✅ Enhances erections
✅ Delays ejaculation
✅ Improves sexual satisfaction
✅ Treats erectile dysfunction
✅ Enhances sexual performance
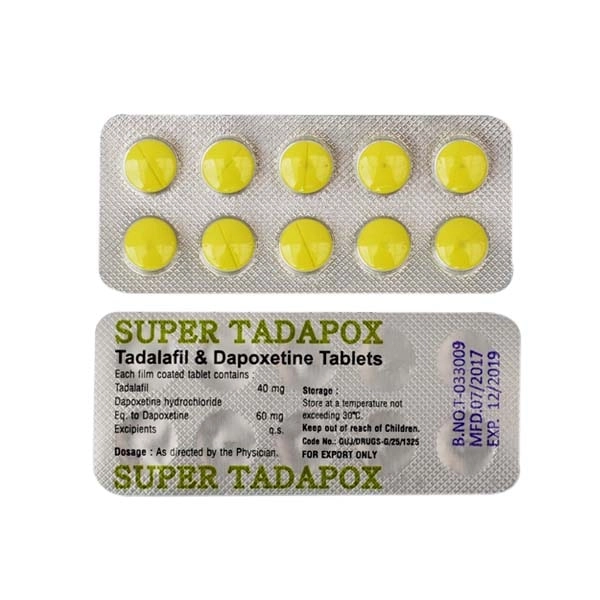
Tadapox contains Tadalafil and Dapoxetine.
Product Overview
Tadapox is a dual-action medication combining Tadalafil and Dapoxetine to effectively treat two prevalent male sexual health concerns: erectile dysfunction (ED) and premature ejaculation (PE). This innovative formulation provides a comprehensive solution for men seeking enhanced sexual performance and prolonged intimate experiences.
Primary Indications
Tadapox is clinically indicated for:
- Erectile Dysfunction Management: Facilitates achieving and sustaining firm erections through improved penile blood circulation
- Premature Ejaculation Control: Significantly extends intravaginal ejaculation latency time (IELT) for more satisfying sexual encounters
Optimal Usage Guidelines
For maximum efficacy and safety:
- Administer one tablet with water 30-60 minutes prior to anticipated sexual activity
- Adhere strictly to the 24-hour dosing interval (maximum one tablet daily)
- Avoid concurrent consumption of alcohol or grapefruit products to prevent potentiation of adverse reactions
- This is an on-demand medication not designed for continuous daily administration
Mechanism of Action
The therapeutic effects are achieved through synergistic pharmacology:
- Tadalafil (20mg): PDE5 inhibition promotes cyclic GMP-mediated smooth muscle relaxation in the corpus cavernosum
- Dapoxetine (60mg): SSRI activity modulates serotonin neurotransmission in the ejaculatory reflex pathway
Dosing Protocol
The standard therapeutic dose consists of one tablet (Tadalafil 20mg/Dapoxetine 60mg) administered orally as required before sexual activity. Dose escalation is contraindicated due to increased adverse event risk. The medication exhibits:
- Onset: 30-60 minutes post-administration
- Duration: Up to 36 hours of erectogenic potential
Therapeutic Advantages
- Dual efficacy for both ED and PE in single-dose administration
- Significant improvement in sexual performance metrics
- Enhanced ejaculatory control and sexual satisfaction
- Prolonged therapeutic window compared to conventional therapies
- Positive impact on sexual confidence and relationship dynamics
Adverse Effect Profile
Most commonly reported transient effects include:
- Cephalalgia (headache)
- Vertigo (dizziness)
- Gastrointestinal discomfort (nausea, dyspepsia)
- Vasodilation-related flushing
Important Considerations
Clinically significant precautions involve:
- Absolute contraindication with nitrate therapy (risk of profound hypotension)
- Caution required in patients with cardiovascular comorbidities
- Potential pharmacokinetic interactions with CYP3A4 inhibitors/inducers
Safety Contraindications
Tadapox is prohibited for:
- Patients with hypersensitivity to active constituents
- Women and pediatric populations (<18 years)
- Severe hepatic/renal impairment (Child-Pugh C, CrCl <30ml/min)
- History of non-arteritic anterior ischemic optic neuropathy (NAION)
Storage Specifications
Maintain product integrity by:
- Storing at controlled room temperature (15-30°C)
- Protecting from moisture and light exposure
- Keeping in original packaging until use
- Properly disposing of expired medication
Medical Disclaimer
This information serves educational purposes only and does not constitute medical advice. While we strive for accuracy, this content should not replace professional medical consultation. Always:
- Disclose full medical history to your physician
- Review potential drug interactions
- Report any adverse effects promptly
The physician-patient relationship remains paramount for safe medication use.
| Strength | 20+60 mg |
|---|---|
| Quantity | 10 Tablet/s, 30 Tablet/s, 60 Tablet/s, 90 Tablet/s, 180 Tablet/s |
 Tadapox is a combination medication that contains two active ingredients: Tadalafil and Dapoxetine. Tadalafil is used to treat erectile dysfunction (ED) by increasing blood flow to the penis, while Dapoxetine is used to treat premature ejaculation (PE) by delaying ejaculation. Together, they provide a dual-action treatment for men experiencing both conditions. Tadapox is typically taken as needed, approximately 30-60 minutes before sexual activity. It is important to follow the prescribed dosage and consult a healthcare provider before use, especially if you have underlying health conditions or are taking other medications.
Tadapox is a combination medication that contains two active ingredients: Tadalafil and Dapoxetine. Tadalafil is used to treat erectile dysfunction (ED) by increasing blood flow to the penis, while Dapoxetine is used to treat premature ejaculation (PE) by delaying ejaculation. Together, they provide a dual-action treatment for men experiencing both conditions. Tadapox is typically taken as needed, approximately 30-60 minutes before sexual activity. It is important to follow the prescribed dosage and consult a healthcare provider before use, especially if you have underlying health conditions or are taking other medications.




































Reviews
There are no reviews yet