Tadarise is a medication primarily used to treat erectile dysfunction (ED) in men. It contains the active ingredient Tadalafil, which works by increasing blood flow to the penis, enabling a firm and lasting erection when sexually stimulated. Tadarise is available in various strengths and is often prescribed for both ED and benign prostatic hyperplasia (BPH). It should be taken as directed by a healthcare professional and is not intended for use by women or children. Common side effects may include headaches, indigestion, and muscle aches. Always consult a doctor before use.
✅ Improves erections
✅ Enhances sexual performance
✅ Increases sexual satisfaction
✅ Treats erectile dysfunction
✅ Boosts libido
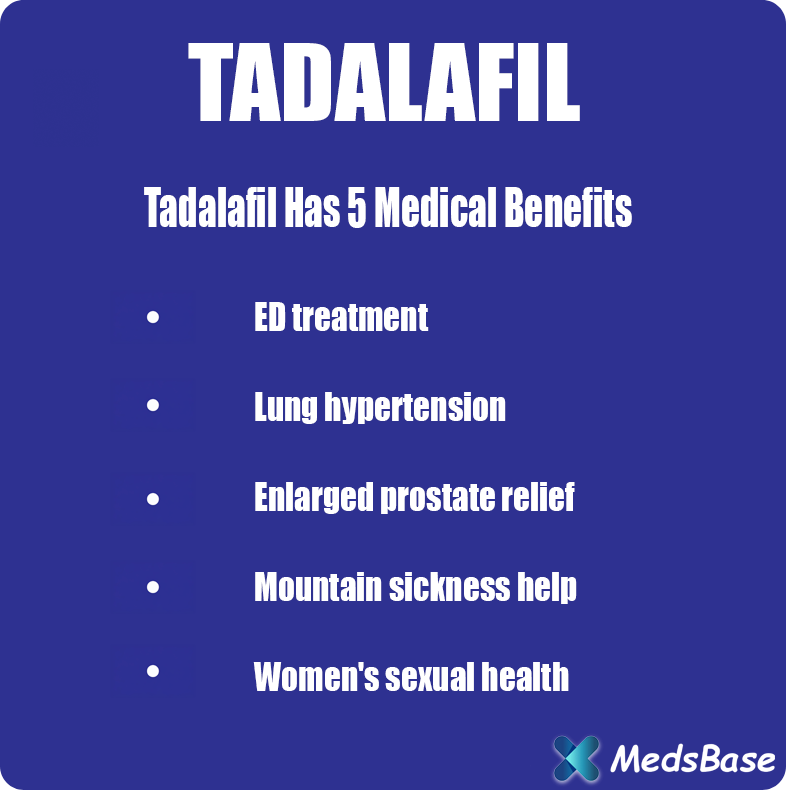
Tadarise contains Tadalafil.
Product Overview
Tadarise is a pharmaceutical formulation containing Tadalafil as its active component, a selective inhibitor of phosphodiesterase type 5 (PDE5). Designed to combat erectile dysfunction (ED), this medication effectively addresses the persistent inability to attain or sustain an erection adequate for satisfactory sexual performance. Tadarise provides a clinically validated solution for men seeking to restore sexual vitality and improve intimate relationships.
Therapeutic Applications
Tadarise is clinically indicated for the treatment of erectile dysfunction (ED), a medical condition marked by the consistent inability to develop or maintain penile rigidity sufficient for sexual intercourse. Through its mechanism of enhancing penile blood circulation during sexual arousal, Tadarise facilitates reliable erection achievement and maintenance, thereby improving sexual function and partner satisfaction.
Administration Guidelines
For optimal results with Tadarise:
- Ingest one tablet orally with adequate water, preferably prior to meals
- The initial recommended dosage is 10 mg, administered approximately 30-60 minutes before anticipated sexual activity
- Refrain from excessive alcohol consumption or heavy meals prior to administration as these may diminish therapeutic efficacy
- The maximum daily dosage should not exceed one tablet (20 mg)
Mechanism of Action
Tadarise exerts its therapeutic effect through selective inhibition of phosphodiesterase type 5 (PDE5) enzyme activity. This inhibition prevents the degradation of cyclic guanosine monophosphate (cGMP) in penile tissues, resulting in vascular smooth muscle relaxation, enhanced blood flow to the corpus cavernosum, and consequent erection development when combined with sexual stimulation.
Dosage Protocol
The standard initial dose of Tadarise is 10 mg, administered orally as required before sexual activity. Dosage may be titrated to 20 mg or reduced to 5 mg based on individual therapeutic response and tolerability. The medication should not be administered more frequently than once per 24-hour period.
Therapeutic Advantages
- Effectively restores erectile function and improves sexual performance
- Demonstrates rapid onset of action with prolonged duration (up to 36 hours)
- Provides dosing flexibility for spontaneous sexual activity
- Enhances sexual confidence and relationship intimacy
- Suitable for adult males across various age groups experiencing ED
Adverse Effects
Common adverse reactions associated with Tadarise may include:
- Cephalalgia (headache)
- Facial flushing
- Dyspepsia
- Musculoskeletal discomfort
- Nasal mucosal congestion
These transient effects typically resolve spontaneously within several hours. Persistent or severe symptoms warrant medical consultation.
Precautions
Clinical considerations regarding Tadarise include potential pharmacokinetic interactions with nitrate medications and alpha-adrenergic blockers, which may precipitate hypotensive episodes. Patients with cardiovascular conditions, hypotension, or other systemic diseases should obtain medical evaluation prior to initiating therapy.
Contraindications
Tadarise is contraindicated in patients with known hypersensitivity to Tadalafil or formulation excipients. The medication is not indicated for pediatric use or female patients. Individuals with significant cardiovascular disease, hepatic/renal impairment, or ocular pathologies require physician consultation before use. Concomitant consumption of grapefruit products should be avoided due to potential pharmacokinetic interactions.
Storage Conditions
Maintain Tadarise tablets in their original packaging at controlled room temperature (15-30°C), protected from humidity and direct heat sources. Keep securely stored away from pediatric access. Do not utilize beyond the expiration date indicated on the packaging.
Medical Disclaimer
The information provided herein is intended for educational purposes only and represents expert-reviewed, evidence-based data. This content should not substitute for professional medical advice, diagnosis, or treatment. The complete safety profile, potential drug interactions, and contraindications may not be fully detailed. Always consult with a qualified healthcare provider regarding any medical condition or therapeutic regimen. This information aims to complement, not replace, the critical physician-patient relationship.
| Strength | 5 mg, 10 mg, 20 mg, 40 mg |
|---|---|
| Quantity | 30 Tablet/s, 60 Tablet/s, 90 Tablet/s, 180 Tablet/s |